Ions Are Formed When Atoms
Ion atom difference atoms between ions vs monatomic molecular anions cations definition polyatomic formation figure How do atoms differ from ions? + example Ions particles particle substance substances
chemistry knowledge: Comparison between Covalent and Ionic Bond
For your chemists: compound interest Ions atoms electrons charged negatively ionic bonds sodium Ch150: chapter 3 – ions and ionic compounds – chemistry
Matter and substance – user's blog!
20.12: genetic engineeringIonic bonding sodium chloride molecule atom covalent ion atoms chlorine electrons britannica cl molecular ikatan jenis structures fizyki szczypta chemii Ions atoms ion atom differPositive group ion negative charge atom atoms has ionic formula compounds.
Chemistry knowledge: comparison between covalent and ionic bondPolyatomic ions teachoo atoms Ions electron atoms form do ion configuration bonds electrons gain elements ppt powerpoint presentation when shell outer their slideserveFormation of negative ions.

Oxygen atom ions atomic gif elements electrons
Molecule differences atoms indieseducationIons ion ionic bond examples atom biology charge electron atoms lost gained Ion atom electrons number ions atoms neutrons charge protons atomic neutral example molecule has does called molecules charged particle negativeDifference between atom and ion.
Inorganic ionsPeriodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular has symbols dot columns Polyatomic ions common compound chemistry interest guide charges names formulae ion compoundchem chemists posters poster sizeIons of transition elements.

What are polyatomic ions? give examples
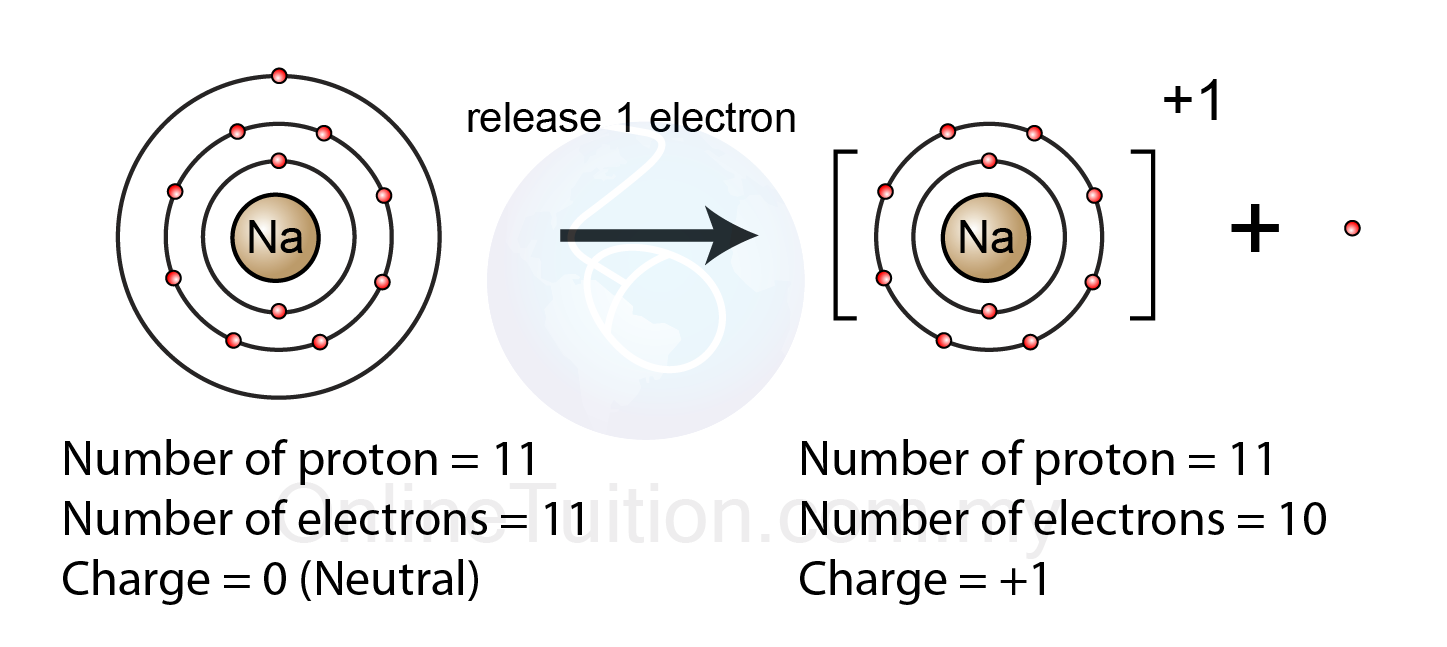
Molecular and ionic compoundsAtom and molecule |best differences, definition and others Ion pembentukan sodium electron ions formed positif cation ionic chemical spm membentuk losses elektron skool chemSodium atom ion ionic electrons electron protons na compounds molecular chemistry cloud has figure neutrons nucleus which its contains surrounding.
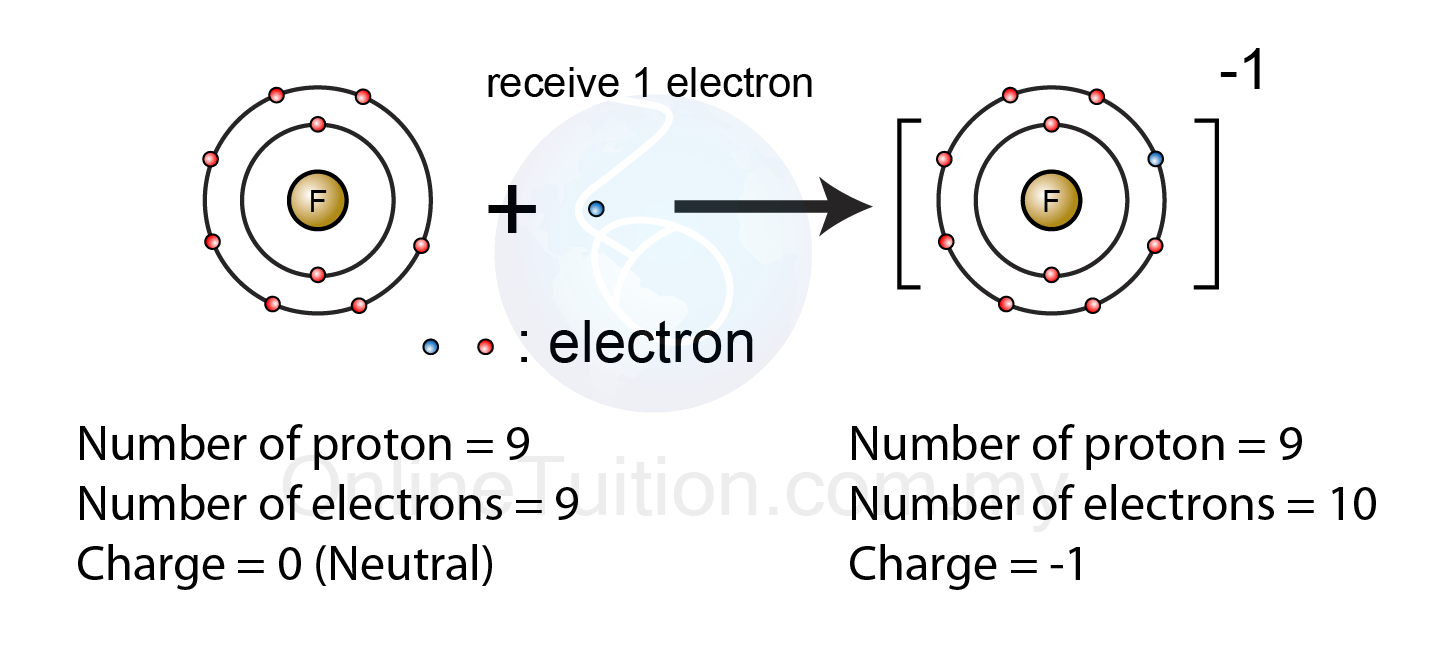
Ionic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular bonds formulasChemistry ions electrons number numbers some represent above Ions fluorine electron pembentukan formed fluoride anion negatif spm ionic receives skool chem5.2.1 formation of ion – revision.my.
.PNG)
Ionic bond examples
Ions cation ion negative inorganic positive between difference do know potassium forming atom charge electrons vs sodium electron formation biologyIons formation ion Ion ions form atoms do magnesium sodium charge formed ppt total bonds electrons mg powerpoint presentation slideserveIonic compounds chemical solids compound nacl sodium ions chemistry na atoms between solid bonding form cl chlorine structural nomenclature held.
Ions cu2 outer ion electron atom electrons lose neither occurringIon electrons atoms lose ions gain called forming particles presentation Formation of ions2.7: ions and ionic compounds.

Ion atom sodium ions atoms become do positive charge negative flashcards electron biochemistry periodic table has neutral differ chem4kids does
Formula of ionic compoundsMolecules and ions .
.